
Basic Inventory Control 5.0.113 serial key or number
Basic Inventory Control 5.0.113 serial key or number
Enhance Inventory with Serial Number Tracking
Inventory is one of the largest assets that many businesses own. Keeping a close eye on a large inventory can be a challenging task, so it is important to have an appropriate strategy to keep track of all the “loose ends”. Implementing an inventory management system with serial number tracking capabilities is a good way to maintain control over inventory by assigning a serial number to each product, part or material that your business produces or holds in stock.
Once a serial number inventory system is in play, your team can track any given item throughout the production process. Let’s take a look at what exactly serial numbers are, how they can be implemented and why they benefit businesses with a large inventory function.
Serial numbers are a unique and individual-item-specific number
A serial number is specifically assigned to a particular item of inventory. They differ from other types of identifying numbers such as product codes and batch and lot numbers. By way of example, a motorbike manufacturer might assign one product code to a particular model of motorcycle, which may be produced at number of the company’s factories. Every unit of that model will share the same product code.
Motorbikes produced during a given period of time at a given production location will then get a lot number that represents this information. Every unit produced at that time and place will share that same lot or batch number.
But the serial number is different – each and every motorbike will be assigned a serial number that uniquely identifies that motorbike. A common, everyday life example of this is the Vehicle Identification Number, or VIN. While the first part of a VIN is a lot number (and thus common to many units), the second part is a serial number that identifies the exact car among others of the same model.
Not every type of product will need to be identified down to the very unit. Expensive items such as IT equipment, electronics, automobiles and jewelry often bear a serial number, while for inexpensive or basic products, a part or model number might suffice.
Implementing serial numbers
Some items will come marked with manufacturer serial numbers already; generally, it will be perfectly appropriate to use the supplier’s serial number. Not every supplier will provide items with serial numbers, but is a relatively simple matter to generate a serial number and mark it on to each item.
The information gift of serialized inventory
A serial number inventory system can unlock a wealth of information about inventory items. Businesses with serialized inventory can see a part or product as it moves through the supply chain. Useful data become available, such as the inventory turnover rate, allowing your business to make better, more informed decisions when purchasing inventory in the future.
Moreover, a serial number can be listed against a particular sale that then appears in a customer’s record. This means that you are able to view the after-sales service history of a particular item as well as track other aspects of its history such as the item’s warranty status.
Inventory numbers also make the stressful process of a product recall much easier for both suppliers and customers. Unleashed supports serial number tracking for products, making it easier than ever to implement a serialized inventory system.

Article by Melanie Chan in collaboration with our team of Unleashed Software inventory and business specialists. Melanie has been writing about inventory management for the past three years. When not writing about inventory management, you can find her eating her way through Auckland.
------- RESEARCH REPORTING SERIES Research reports of the Office of Research and Development, U.S. Environmental Protection Agency, have been grouped into nine series. These nine broad cate- gories were established to facilitate further development and application of en- vironmental technology. Elimination of traditional grouping was consciously planned to foster technology transfer and a maximum interface in related fields. The nine series are: 1. Environmental Health Effects Research 2. Environmental Protection Technology 3. Ecological Research 4. Environmental Monitoring 5. Socioeconomic Environmental Studies 6. Scientific and Technical Assessment Reports (STAR) 7. Interagency Energy-Environment Research and Development 8. "Special" Reports 9. Miscellaneous Reports This report has been assigned to the INTERAGENCY ENERGY-ENVIRONMENT - RESEARCH AND DEVELOPMENT series. Reports in this series result from the -effort funded under the 17-agency Federal Energy/Environment Research and Development Program. These studies plate to EPA's mission to protect the public health and welfare from adverse effects of pollutants associated with energy sys- tems. The goal of the Program is to pssure the rapid development of domestic/ energy supplies in an environmentally-compatible manner by providing the nec- essary environmental data and control technology. Investigations include analy- ses of the transport of energy-related pollutants and their health and ecological effects; assessments of, and development of, control technologies for energy systems; and integrated assessments of a wide'range of energy-related environ- mental issues. EPA REVIEW NOTICE This report has been reviewed by the participating Federal Agencies, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Government, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This document is available to the public through the National Technical Informa- tion Service, Springfield, Virginia 22161.
------- EPA-600/7-80-083 April 1980 Sulfur Dioxide Oxidation in Scrubber Systems by J.L Hudson University of (Virginia Department of Chemical Engineering Charlottesville, Virginia 22901 Grant No. R805227 Program Element No. EHE624 EPA Project Officer: Robert H. Borgwardt Industrial Environmental Research Laboratory Office of Environmental Engineering and Technology Research Triangle Park, NC 27711 Prepared for U.S. ENVIRONMENTAL PROTECTION AGENCY Office of Research and Development Washington, DC 20460 I'ml
------- ABSTRACT This study relates the liquid phase oxidation of bisulfite and sulfite anions to sulfate to the conditions in lime/limestone scrubbing systems such as those used for removal of sulfur dioxide from power plant stack gases, Experiments were carried out for the oxidation in both calcium sulfite and sodium sulfite clear solutions and in calcium sulfite slurries. In the slurry studies, both the rate of chemical reaction and the rate of solid to liquid mass transfer were investigated, but with both slurries and clear solutions, the experiments were run so that gas to liquid transfer of oxygen was not a limiting resistance. A mathematical model for the dissolution of solid particles and liquid phase chemical reaction was developed in conjunc- tion with these experimental results, The oxidation was carried out over a pH range of 4.0 to 5.5 (although some experiments were at pH 6 and pH 11), at temperatures of 25°C to 50°C, and in the presence of iron and manganese catalysts and several organic acid inhibitors. Batch reactors were used for the slurry studies and for the slower clear solution oxidations while a flow reactor was employed for faster clear solution reactions. Although under special combinations of catalyst, pH, and organic acid, the order was as low as one or rose to two, the homogenous chemical oxidation rate of calcium sulfite oxidation is 1.5 prder over most of the range of conditions. The rate increases strongly with increasing pH. Of the organic acids studied, glycolic is the strongest inhibitor, followed by adipic and succinic. Citric and acetic acids are less inhibitory than the others. Both manganese and iron catalyze the reaction even in the presence of the organic acid inhibitors. Oxygen concentration varied over a large range; its order is 0.5 in the rate expression for the manganese catalyzed oxidation at higher pH. In a three phase slurry oxidation the overall reaction of sulfite to sulfate declines with increasing pH; this decrease is caused by the sharply reduced calcium sulfite solubility with increasing pH. This calcium sulfite solubility was determined in independent experiments. The results of the mathematical model for the dissolution and reaction of calcium sulfite particles compare well with results of the three phase slurry oxidations. ii
------- CONTENTS Abstract i:L Figures v Tables viii 1. Introduction . . . 1 A. General Considerations * 1 B. Background Literature 3 2. Experiments 32 A. Apparatus 33 1. Batch Reactor 33 2. Flow Reactor , 37 B. Procedure 44 1. Rate from Concentration Measurements 44 2. Rate from pH Measurements 46 3. Rates from Temperature Measurements 46 C. Analysis of Calcium Sulfite 48 D. Equilibrium Relationship for S 44Species 50 E. lodometric Titration 50 F. Analysis of Data 52 1. Analysis of a Single Experiment. 52 2. Multiple Regression Analysis 53 3. Analysis of pH Method Data 61 4. Analysis of Flow-Thermal Method 64 3. Oxidation in Calcium Sulf ite Solutions 69 A. CaSO- Oxidation (No organic acids) 70 B. CaSO^ Oxidation in the Presence of Organic Acids.. 71 1. Succinic Acid 83 a. Effect of pH on the rate of oxidation.... 83 b. Effect of succinic acid concentration.... 85 2. Adipic Acid 90 3. Glycolic Acid 90 4. Comparison of Rates of Oxidation with Succinic Acid, Adipic Acid, Glycolic Acid, Citric Acid, and Acetic Acid 96 5. Effect of CatalystsManganese and Iron 107 a. Succinic Acid 107 b. Glycolic Acid Ill C. Reaction in Liquor from Penberthy Oxidation Runs at Shawnee 118 D. Dependence of Oxidation Rate on Stirring Speed and Oxygen Flow Rate 122 E. Rate of CaSO^ Oxidation at High Catalyst Concentra- tions by Flow-Thermal Method 133 iil
------- 4. Oxidation in Slurries 139 A. Experiment 140 1. Solubility Studies 140 a. Theoretical Analysis 140 b. Experimental Results 141 2. Oxidation in Calcium Sulfite Slurries 143 3. Oxygen Flow Rate Studies 143 4. pH and Temperature Studies 146 5. Slurry Density Studies 153 6. Catalyzed Slurry Oxidation Studies 153 7. Liquid Phase Slurry Behavior 161 8. pH Behavior During Reaction 161 9. Slurry Reactions with and without pH Controller 161 10. Liquid phase Catalyst Studies 171 B. Mathematical Model 171 1. General Description of Model 171 2. Physical Description of Particles 174 a. Electron Micrograph 174 b. Particle Size Distribution 175 3. Derivation of the Spherical Model. 175 4. Solutions of the Spherical Model. 179 5. Derivation of the Flat Plate Model 192 5. Oxidation in Sodium Sulfite Solutions 194 A. Na-SO^ Oxidation . . . 195 1. Manganese Catalyst 195 B. ^280^ Oxidation with Succinic Acid Buffer 195 1. Iron Catalyst 195 2. Manganese Catalyst 208 3. Mixed Catalysts. . 217 C. Rate of Na.SO- Solution Oxidation by the Flow-Thermal Method 221 6. Conclusion 227 References . . . 233 iv
------- FIGURES Number Page 1-1 Effect of pH on the Relative Concentrations of Sulfur (4+) Species in Solution .4 1-2 Electron Micrograph of Calcium Sulfite Particles - Sulfite and Sulfate Particles . 8 1-3 Electron Micrograph of Calcium Sulfite Particle Individual Agglomerate 8 1-4 Comparison of Gladkii's Figure 6 with Figure 5 23 1-5 Comparison of Slurry Oxidations at 50°C. . . 24 1-6 Comparison of Gladkii's Figure 6 with Soviet Slurry Oxidation Rate Expression 26 1-7 Determination of Soviet Reaction Order .......... 27 1-8 Comparison of Slurry Oxidations at 40°C 29 2-1 Experimental Apparatus 34 2-2 Reaction Vessel 35 2-3 Tubular Flow Reactor 38 2-4 REPORT and CURVIT Programs 54 2-5 Element of Reacting Fluid 66 2-6 Sample Output for Flow Reactor 68 3-1 Rate of Calcium Slufite Oxidation; no added catalyst ... 72 3-2 Rate of Calcium Sulfite Oxidation; [Mn] = 0.6 ppm 76 3-3 Rate of Calcium Sulfite Oxidation; [Mn] = 0.53 ppm, [Fe] = 1.02 ppm . 77 3-4 Rate of Calcium Sulfite Oxidation; variable [Mn] 79 3-5 Rate of Calcium Sulfite Oxidation; variable [Mn], ([S ] = 0.0037 mol/£) 80 3-6 Effect of Temperature on the Rate Constant ........ 81 3-7 Effect of pH on Rate of Reaction 86 3-8 Concentration Sulfur (4+) Versus Time for Various Concentrations of Succinic Acid ..... 87 3-9 Concentration Sulfur (4+) Versus Time, Run 566, 0.2M Succinic Acid 88 3-10 The Inhibition of the Sulfite Oxidation Due to Succinic Acid 89 3-11 Initial Rate Versus Succinic Acid Concentration 91 3-12 The Inhibition of the Sulfite Oxidation Due to Adipic Acid. /-+ (^ 3-13 Concentration S vs Time for Various Concentrations of Glycolic Acid, pH = 4.0 97 3-14 Inhibition of the Sulfite Oxidation due to Glycolic Acid . 98 3-15 The pH Effect on the Inhibition of the Sulfite Oxidation Due to Glycolic Acid 99 3-16 Effect of Glycolic Acid on Rate of Oxidation of CaSO , at T=50°C, pH=4.0, [S ]=0.01M ....... 102 3-17 Comparative Strength of Various Organic Acids as Inhibitors at 0.2M 103 v
------- FIGURES (continued) 3-18 Comparative Strength of Various Organic Acids as Inhibitors at 0.01M 104 3-19 Sulfur (4+) Concentration vs Time for Various Mn Concentrations HQ 3-20 Effect of Mn on Oxidation Rate+of Succinic Acid Buffered CaSO Solutions for [S ]=0.01M, T=50°C, pH=5.0 ... 113 3-21 Concentration Sulfur (4+) vs Time, 1000 ppm Glycolic Acid, Various Concentrations of Mn 114 3-22 Effect of Mn Addition to Glycolic Acid Inhibited CaSO Oxidation; rate at [S ] = 0.01M 116 3-23 Effect of Mn Addition to Glycolic Acid Inhibited CaSO, Oxidation; Rate at [S ] = 0.01 M 117 3-24 Stirring Speed = 215 rpm '.'.'. 123 3-25 Stirring Sp,eed = 1800 rpm *'-.'' 124 3-26 Rate at [S ] = 0.01 M vs Stirring Speed . .' .' .' .' . .' .' .' 128 3-27 1.4 Order Rate Constant vs Mn Concentration in CaSO., Filtrate Oxidations ....... 132 3-28 Representative T vs t Results for CaSO- Oxidation. .'.'.'.' 134 3-29 Effect of [Mn] on CaS03 Oxidation Rate 136 4-1 Solubility of Calcium Sulfite at 40°C. . 142 4-2 Effect of Slurry Density on Oxidation Rate ........ 144 4-3 Typical Slurry Reaction Curve 145 4-4 Effect of Mn Catalyst on Slurry Oxidation Rate ...... 147 4-5 Slurry Oxidation, pH = 4.5 ' 143 4-6 Slurry Oxidation, pH = 4.7 "'.'.'.'.'.'. 149 4-7 Slurry Oxidation, pH = 5.0 .............. . . 150 4-8 Effect of pH on Slurry Oxidation Rates ^ 4-9 Slurry Oxidation, pH = 4.5 ' 154 4-10 Slurry Oxidation, pH = 4.5 '.'.'.'.'.'.'. 155 4-11 Catalyzed and Uncatalyzed Oxidations at 50°C 158 4-12 Effect of Catalyst on pH Behavior (40°C) ......... 159 4-13 Effect of Catalyst on pH Behavior (50°C) ......... 160 4-14 Slurry Oxidation, 200 ppm Mn, pH = 5.0 '. 164 4-15 Slurry Oxidation, 200 ppm Mn, pH = 4.5 165 4-16 Slurry Oxidation, 200 ppm, pH = 5.0 166 4-17 Slurry Oxidation, pH = 5.0 . . '.'.'. 167 4-18 pH Behavior During Slurry Oxidations; Effect of Slurry Density . . . \ _ 168 4-19 pH Behavior During Slurry Oxidations; Effect of Initial . PH 169 4-20 pH Behavior During Slurry Oxidations; Highly Catalyzed Runs 170 4-21 Slurry Oxidation with No Mn Added 172 4-22 Liquid Phase Catalyst Behavior During Slurry Oxidations. . 173 4-23 B-30 Program and Sample Calculation 181 4-24 Determination of ke from Highly Catalyzed Slurry Data (2000 ppm Mn add^d) 187 4-25 Comparison of Total S from Model to Experimental Slurry Oxidation Data for No Added Mn 188 VI
------- FIGURES (continued) 4-26 Comparison of Model to Experimental Data for 6.66 ppm Mn Added. . 189 4-27 Comparison of Model to Experimental Data for 200 ppm Mn Added 19° 4-28 Comparison of Liquid S from Model to Experimental Slurry Oxidation Data for No M£+ Added 191 4-29 Computer Predicted Liquid [S ] Profiles 193 5-1 Dependence of Rate on Sulfite Concentration 197 5-2 Dependence of Rate on Manganese Concentration 198 5-3 Sulfur S Concentration vs Time for Iron Catalyzed Na2S03 Oxidations 20° 5-4 Rate at [S ] = 0.028 mol/£ v£ Concentration Iron Added for Sodium Sulfite Oxidations 203 5-5 Sulfur (4+) Concentration vs Time for Iron Catalyzed Oxidations at Various Temperatures Using Na^O- . . . 204 5-6 Activation Energy Determination for Fe Catalyzed Na2Su3 Oxidations ' 206 5-7 Rate at [S ] = 0.028 M vs Concentration Fe 210 5-8 Sulfur (4+) Concentration vs Time for Manganese Catalyzed Oxidations of Na9SO« Solutions 213 5-9 Rate at [S ] = 0.02S M vs Concentration Mn 215 5-10 Reduced Sulfur (4+) Concentration vs Time for Comparison of Oxidation Reactions 218 5-11 Comparison of Mn Added Only to Oxygen Side or Only to Sulfur Side 226 vii
------- LIST OF TABLES Number 1-1 Fly Ash Analysis 6 1-2 Limestone Analysis 9 1-3 Comparison of Literature of Sulfite Dioxide Oxidation. . . 15 2-1 Analysis of Calcium Sulfite Solid 49 3-1 Rate of Calcium Sulfite Oxidation; No added catalyst (PH - 4.6) 73 3-2 Rate of Calcium Sulfite Oxidation; [Mn] = 0.6 ppm 74 3-3 Rate of Calcium Sulfite Oxidation; Added Mn and Fe . . . . 75 3-4 Rate of Calcium Sulfite Oxidation; variable [Mn] 78 3-5 Effect of Temperature on Rate Constant 82 3-6 Effect of pH on Succinic Acid Buffered CaSO» Solutions . . 84 3-7 Effect of Succinic Acid on Oxidation Rate. 92 3-8 Results of Order and Rate Determination Analysis of Data for Calcium Sulfite Oxidation with Varying Concentra- tions of Adipic Acid 93 3-9 Results of Order and Rate Determination Analysis of Data for Calcium Sulfite Oxidations with Varying Concen- trations of Glycolic Acid Added 95 3-10 Effect of Glycolic Acid on Oxidation Rate of Calcium Sulfite 100 3-11 Results of Order and Rate Determination Analysis of Data for Calcium Sulfite Oxidations with Varying Concen- trations of Glycolic Acid Added 101 3-12 Effect of Organic Acids on Oxidation Rate of Calcium Sulfite 105 3-13 Catalyst Impurity Levels in CaSO., Solutions 108 3-14 Effect of Mn Impurity on Oxidation Rate 109 3-15 Effect of Mn Concentration on Oxidation Rate of Succinic Acid Buffered CaS03 Solutions 112 3-16 Effect of Manganese Addition to Glycolic Acid Inhibited Runs . 115 3-17 Oxidation of Sulfite Solutions 119 3-18 Supplemental Analysis of Shawnee Sample 121 3-19 Effect of Stirring Speed - No added catalyst 125 3-20 Effect of Stirring Speed on Reaction Rate Constant - 0.5 ppm Mn. . 127 3-21 Effect of Oxygen Flow Rate on Oxidation in Unsaturated Solutions . 129 3-22 Results of Filtrate Oxidations; pH,- = 4.5, T = 40°C. . . . 131 3-23 Effect of High Mn Concentration on CaSO.. Oxidation Rate . . 137 3-24 Effect of pH on the Rate of Mn Catalyzed CaS03 Oxidation. . 138 4-1 Results of Studies Showing Effect of Initial pH on Oxidation 152 viii
------- LIST OF TABLES (continued) 4-2 Effect of Slurry Load on Oxidation Rate at 40°C 156 4-3 Effect of Catalyst on Slurry Oxidation Rate 157 4-4 Experiments Made in Mn Catalyst Study 162 4-5 Experimental Conditions Used in Slurry Oxidations Shown in Figures 4-5 to 4-7, 4-9, 4-10, 4-14 to 4-17 163 4-6 Size Distribution of Calcium Sulfite Particles (Coulter Method) 176 5-1 Na SO Oxidation 196 5-2 Results of Sodium Sulfite Oxidation with Iron Catalyst Added 202 5-3 Results of Iron Catalyzed Reactions at Various Temperatures Using Sodium Sulfite 205 5-4 Results of Sodium Sulfite Oxidations with Varying Initial Sulfite Concentration and 5 ppm Iron Added 207 5-5 MULTREG Results for Iron Catalyzed Na2S03 Oxidations. ... 209 5-6 Results of Sodium Sulfite Oxidations with Manganese Added . 211 5-7 Regression Analysis of Manganese Catalyzed Na^O^ Oxidations 2l6 5-8 Results of Sodium Sulfite Oxidations with Iron and Manganese Added. 5-9 Multiple Regression Results for Mixed Catalyst Sodium Sulfite Oxidations 22° 5-10 Effect of [Mn] on Na2SOa Oxidation Rate 223 5-11 Effect of pH on Na2S03 Dxidation Rate 224 ix
------- SECTION 1 I. Introduction A. General Considerations B. Background Literature INTRODUCTION A. General Considerations In the removal of sulfur oxides from stack gases by any of the scrubbing methods, a fraction of the sulfur compounds is oxidized to sulfates. This oxidation occurs whether the scrubbing agent is a slurry (lime/limestone systems) or a clear solution (double alkali or other such systems). Further- more, the oxidation occurs not only in the absorber, but also in other sec- tions of the system, such as in the hold tanks. In lime/limestone scrubbing systems S02 oxidation is important for sev- eral reasons. (The species actually taking part in the reaction is either a bisulfite or sulfite ion depending on the pH of the solution; however, it is convenient and it is common practice to refer to sulfur dioxide or calcium sulfite oxidation.) The oxidation in the scrubbing system can increase the degree of supersaturation of calcium sulfate, leading to an increase in the rate of gypsum scale formation (183, 30). Thus, it is important to limit calcium sulfite oxidation in some systems. On the other hand, calcium sulfate is preferable to calcium sulfite from the standpoint of solids disposal since the sulfate has a higher settling velocity than the sulfite and the sulfate also has a higher compaction and a lower chemical oxygen demand (31-36). The latter is important from water pollution considerations. Therefore, in some
------- limestone scrubbing systems it may be desirable to promote or inhibit oxi- dation. Oxidation in lime/limestone systems, both in the scrubber and in the hold tanks, is a very complicated process. The rate of oxidation can depend on chemical kinetics since the rates of liquid phase reactions of sulfite ion and bisulfite ion vary with concentration, with the presence of catalysts or inhibitors, and with the type of oxidizing agents, viz., oxygen or nitrogen oxides. (It should be noted that very small concentrations of some catalysts can influence the reaction greatly and that, therefore, many reactions which are supposedly being run without catalysts are in fact being catalyzed by impurities present.) The rate of oxidation is also influenced by phase and chemical equilibria. There are three phases present in the scrubbing system, solid, liquid, and gas. Both the phase equilibria and chemical equilibria are strongly dependent on pH. For example, lowering the pH in the presence of calicium sulfite increases the solubility and, therefore, increases the con- centration of dissolved sulfur containing species. This can increase the rate of oxidation under some conditions, particularly, say, in a hold tank. How- ever, a lowering of the pH of scrubbing liquor in the absorber converts sul- fite ion to bisulfite ion; this would lower the rate of oxidation since sulfite ion is oxidized much more quickly than bisulfite ion in a clear liquor (but not in a slurry where solubility effects are important). Finally, the rate of oxidation can be influenced or controlled by mass transfer, viz., by the rate of transfer of oxygen to the liquor and by the rate of dissolution of solid particles. The complex sulfur dioxide oxidation process was broken down into steps and each step studied separately. The oxidation of calcium sulfite and sodium sulfite clear solutions was inventigated as a function of manganese and iron
------- catalysts, pH, temperature, and organic acid inhibitors. The rate of dissolu- tion of calcium sulfite particles in a stirred liquid was determined and this information, along with the kinetic rate results, were used as a basis for a mathematical model for the dissolution and reaction of calcium sulfite parti- cles . Measurements were then made on the rate of oxidation in calcium sulfite slurries and the results compared to predictions of the model. B. Background Literature Atmospheric sulfur dioxide affects our lives in many ways. It is a health hazard (9, 10), corroder of structures (9.4) and equipment, visibility impairer (106), and habitat modifier (88, 166). Man's activities of smelting and burning fossil fuels among others have significantly altered the world sulfur budget (97), threatened his health (10, 57), and now require control (60, 61). Control methods include: wet scrubbing, dry sorbents (furnace injection) (144, 161), catalytic oxidation (5, 27, 37, 38, 73, 121, 123, 146, 164), dry filters (and baghouse add-ons) (1), and fuel precleaning (111). Today, wet scrubbing is the most widespread method (59) for cleaning S02 from stationary sources. When the S02 enters any scrubbing liquor, the fol- lowing equilibria are set up (154): S02 + H20^=^S02-mH20^=^HSO; + H+^=^So|" + H+ low *-pH *" high These relationships are shown graphically in Figure 1-1. Water-only scrubbing is ineffective. Removing the H+ in the last two equilibria above effectively shifts the concentrations to the right, thereby sequestering the sulfur dioxide as bisulfite on sulfite anions. All of the scrubbing methods
------- 1.0. 0.0- 0.6 Hole Fraction 0.4- 0.2-H 0.0. HS03 Conditions 40°C 0.02 M Na SO '""'3 1 T pH T 8 S03 2- 10 Figure 1-1. Effect of pH on the Relati.ve Concentrations of Sulfur (4+) Species in Solution.
------- turn on this principle of tying up hydronium ion by the addition of many kinds of alkalis (eg. CaC03, CaO, NaOH, NH3). The resulting sulfite/bisulfite solu- tion can be discarded (these are "throwaway" processes) or regenerated (132, 177). Overall, the scrubbing process consists of: Gas-Liquid mass transfer from the stack gas, equilibria and reaction in the scrubbing mixture, modula- tion by Solid-Liquid transfer of absorbents (and possible Liquid-Solid trans- fer of products). The Gas-Liquid mass transfer is accomplished in several ways (90): spray tower, venturi (89, 98, 175), packed bed (159) and turbulent contact (moving ball) absorber (113). The efficiency of the G-L contacting step has been the subject of much study to assess major resistances (120, 178) and control them (142). Since S02 is chemically transformed as it enters (102) the liquid surface (from bubbles (104) or surface films (11, 46, 58)), the rates are best treated as absorption with reaction and described by parallel approaches viz., film theory (47, 85, 157), penetration theory (40, 41), or surface renewal (51, 52, 143). From coal fired boilers, stack gas entering the scrubber carries (53) '4 to 7% 02; various oxides of nitrogen; C02, CO, and organic residues; as well as inorganic residues entrained as fly ash (including chloride (29, 114) and metals (105) as well as inert particulates (64, 77). Some scrubbing methods remove particulates (69) as well as sulfur oxides, but these particulates (inorganic and other coal debris) influence the scrubbing process as catalysts and inhibitors of sulfite oxidation. See Table 1-1. The sulfur oxides rapidly (23) form a potentially reactive mixture des- cribed by the S02 dissociation equilibrium (99). The first ionization con- stant being 1.74 x 10"2 (167), the second 6.24 x 10~8 (189). The equilibria have been studied for S02/water systems (137, 169, 170) and scrubbing systems (124, 144).
------- TABLE 1-1. FLY ASH ANALYSIS Chemical Analysis Component Weight Percent A1203 20-30 Fe203 12-23 CaO 2-7 0.5-1.5 6
------- The most common absorbents (solids) are lime, limestone and dolomite found as crystalline aggregates (see Figures 1-3 and 1-2) and solid mixtures (78). Their solubility has been determined (65, 82) and noted in scrubber operation (29, 70). These soluble minerals provide the carbonates and hydrox- ides to fix the S02 in solutions. An analysis (43, 79) is given in Table 1-2. 2- The dissolved sulfur species HSCL and S03 are subject to oxidation in 2_ solution by dissolved oxygen producing SO^ . This gypsum is produced in lime/ limestone throwaway processes and can lead to supersaturation in all calcium-based processes causing scaling (29, 56, 77). Scale formation can be controlled by additive controlled oxidation (below 20%) (29) and gas pre- treatments. The oxidation occurs along the trajectory of the S02 as it enters the absorbing solution (127). Hydrodynamic conditions (151, 168) and mass transfer to the solution (169, 170) determine the region where reaction occurs. The limit of this region, called the reaction plane, determines what theory best describes the rate of absorption (80, 120). For instance, when the film is large (equal or greater than the diameter of the small particles of solid), dissolution can occur in the reacting zone, further increasing reaction and enhancing absorption (138). One approach to scaling control is limitation of oxidation by the addi- tion of an inhibitor (50, 77, 147). Many organic materials (especially those bearing hydroxyl groups) inhibit the oxidation. This action is not likely to be true catalysis (20, 50, 140), hence the inhibitors may be subject to con- sumption and would require replacement. Factors to consider with regard to inhibitors are: i Inhibitor must be water-soluble. Its actions may be modeled well enough to limit oxidation in part of the system.
------- Figure 1-2. Electron Micrograph of Calcium Sulfite Particles Sulfite and Sulfate Particles Figure 1-3. Electron Micrograph of Calcium Sulfite Particle Individual Agglomerate
------- TABLE 1-2. LIMESTONE ANALYSIS Chemical Analysis Component Weight Percent A12°3 Fe203 MnO CaO Cl Cu Cr As Hg 6.01 0.19 0.06 55.5 0.004 0.00044 0.00011 0.0002 6xlO"6
------- Products formed from inhibitors may also retard (or promote) the oxidation. The contacting method in the scrubber changes the effectiveness of inhibitors (43). Fly ash may contain phenolic organic residues active as inhibitors. Recently organic acids used to enhance limestone solubility were identi- fied as rate retarders. The most extensive set of experiments dealing with the organic acid effect of both the sodium and the calcium sulfite oxidation are reported by Hatfield, Kim and Mullins (81). It was reported that in the sodium system, organic acids promoted the sulfite oxidation with the order being: adipic > glycolic > no acid. However, in the calcium system organic acids were found to reduce the oxidation with the order being: glycolic > adipic > no acid. The results dealing with the calcium system are comparable to the results of the present work, dealing with the comparative inhibitory strength of the organic acids. The relative strength was: glycolic > adipic > succinic > no acid. There are large discrepancies between the current results and those of Hatfield et al. for the rate of oxidation of the sulfite. Hatfield et al. reported that the sulfite oxidation goes toward completion in several hours, but the results of this work show that the sulfite goes to sulfate in a matter of minutes (usually less than 20 minutes even with inhibitors added). Hatfield et al. added CaCO (calcium carbonate) to the reaction mixture for pH control and used an oxygen flow rate of only 20 ml/min. The present exper- iments used the addition of NaOH for pH control and a flow rate of oxygen at 3 A/min to maintain 0^ saturation in the reactor. This suggests that while the CaC03 or the NaOH may have an effect on the sulfite oxidation, the experiments by Hatfield et al. were oxygen limited (controlled by the oxygen flow rate 10
------- rather than the sulfite oxidation kinetics). These workers also determined the rate of reaction to increase with pH, which is in agreement with the current findings. In a recent work Altwicker (8) found hydroquinone caused a changing reduction in the oxidation rate which he attributed to inhibitor in the re- action plane being consumed quicker than it could be replenished, casting doubt on the sulfite method for measuring interfacial area in contactors. The scaling problem is greatest in the scrubber and mist eliminator. By 2- inducing oxidation elsewhere in the system, supersaturation in SO, will not happen. For this reason various approaches to forced oxidation in the holding tank beneath the scrubber have been studied (29, 71). For example: forced oxidation of the calcium sulfite reaction product in both lime and limestone FGD systems has been successfully demonstrated in 10 megawatt prototype units at the Shawnee Test Facility. The oxidized gypsum product (74) results in less disposal volume and settles by an order-of-magnitude faster than the unoxidized material. It filters to better than 80% solids and handles like moist soil compared with the unoxidized material which filters only to about 50 to 60% solids and is thixotropic (83). Improved settling is treated elsewhere as well (110, 124, 132, 161), and good sulfate removal has been discussed (29). The problem of scrubber scaling is eliminated in sodium-based liquors (56, 77, 110, 132, 161), but the added cost of sodium absorbents (49, 69, 110) necessitates regeneration of the "alkali values" of the sodium absorbent usually with a calcium base (lime/limestone) that is discarded. Recovery oriented, sulfur-concentrating processes are usually gaseous (27, 128, 165), but some processes (55, 92) use a liquor, operating by electro- lytic regeneration (92). The most prominent (68, 77, 103) two loop process is 11
------- the double alkali process (dual alkali), featuring (56, 96, 110) efficient, higher pH scrubbing with sodium liquor at low L/G rates, reduced scaling, and thorough limestone utilization, but may have disposal problems. Other companies which have utilized liquid phase oxidation in wet scrub- ber flue gas desulfurization processes include Babcock and Wilcox (magnesia-base wet scrubbing) (13), Wellman-Lord, Stone and Webster/Ionics (63), Aerojet-General Corporation (zinc oxide process) (6,92), and Monsanto (CALSOX system) (87). There are many other systems including ones based on magnesia (100, 158). Many models for the physical and chemical events underlying these removal processes have emerged beginning with the equilibria among the solution ionics (133) and a generalization of the oxidation in solution (109, 153). The real challenge is in formulating (47) the complex equilibria, dissolution rates, absorption rates (40), and reaction rates in a way suitable for computing concentration profiles, removal rates and efficiences, and absorbent effi- ciencies in the scrubber (114) and holding tank (175). Any description requires the reaction kinetics of sulfite oxidation in solution. Indeed, this topic has been the subject of multifaceted research for 132 years yielding sure findings only slowly. The oxidation is a free radical chain process subject to photochemical initiation, the quantum efficiency of which is 5 x 104 (16). The impurity level of the work that produced this figure raises doubt about its validity. Further work shows no influence of UV on the oxidation rate in aerosols of low pH (95). The most careful photochemical study concluded that there is no simple relation between the light absorbed and the rate (112). Experiments using various surfaces and particulate additives (86) found no variation with contact area of catalyst. This well establishes the reac- 12
------- tion as a case of homogeneous catalysis. Other workers (20) who exceeded solubility limits of catalyst suggested the subsequent particles might have provided sites for heterogeneous catalysis, but this is not the case (18). The effect of impurities is dramatic. The sensitivity of the reaction rate to stray metal ions is the hallmark of the reaction, but inorganic anions (188) as well as organic molecules affect the rate (although in the opposite way). In fact, the bane of much experimental work was rubber in the apparatus (48, 115, 140, 171). Pure gum rubber stops the reaction (112) giving evidence that the sulfur vulcanizing chemicals are not the cause. The degree of inhi- bition by hydrocarbons is so sensitive that oxidation has been suggested as a semiquantitative analysis for their presence (152). This sensitivity is an important consideration for operations in rubber-lined slurry handling equip- ment, such as those at Shawnee Valley. -12 The acceleration of the rate by metals is extreme: 10 M copper ion added during sulfite oxidation increased the rate (171). Many metals have been studied: CQ 7, 21, 42, 44, 54, 95, 107, 108, 109, 130, 126, 149, 163, 185, 187 r 7, 17, 21, 66, 84, 95, 109, 150, 140, 172 L»U Mn 21, 42, 48, 75, 86, 93, 95, 179 Fe 21, 39, 84, 86, 93, 95, 140 21, 42, 179 Mg 21, 39 Ni 21, 93, 140 Al Zn 21' 179 Ca 179 xra + 21, 45, 95, 115, 140, 152 JNI14 13
------- General comparisons have tested their relative effectiveness: Co, Ni > Cu > Fe ref 140 Mn > Co, Ni > Fe, Cu > Mg, Zn, Na, NH4+ ref 21 Mn » Zn, Mg > Ca ref 179 Mn > Cu > Fe > Co, NH > Na > uncat. ref 95 329 199 167 49 49 4 3 = Relative strength Mn -\. 7000 x Mg ref 42 The most effective catalysts are clearly manganese and cobalt with iron giving a notable effect. The metal showing the least effect is sodium (21, 91, 95). For this reason, the spectator ion is nearly always chosen as sodium. Am- monium, a constituent in many natural systems, increases the rate of oxida- tion. The probable cause is not catalysis, but rather its effect on pH may merely shift the equilibrium toward sulfite which is most rapidly oxidized. Several workers (20, 45, 136, 150, 152) have considered rionmetallic catalysis and mechanistic interactions of sulfur compounds. The very few experiments have no salient results. One curious observation is that sulfate formed by the reaction has less effect than initially added sulfate upon the reaction rate (111, 134). No satisfactory answer exists (42, 66, 141), but control of the position of the sulfur species equilibrium is one candidate. An early controversy concerned bisulfite oxidation rates increasing with dilution (17, 115, 119). The spur- ious effect was due to decreased oxygen mass transfer at higher concentrations (140). The oxidation in all pH ranges has been studied with wide concentration changes of sulfur species and catalyst and some slight changes of oxygen concentration. Many of the important results in the literature are compared Table 1-3. Although no firm results stand out, the many contrasts serve to 14 on
------- TABLE 1-3. COMPARISON OF THE LITERATURE OF SULFUR DIOXIDE OXIDATION Reference lloalhcr & Toodeve8'1 Coughanowr & Krause"8 Walter1" Powell135 Catipovlc1''2 Chen 6. Barion & Llnek & 108 Alper7 Relnders & Fc"et^ Phillips i Johnson DeWaal & Ok on 54 Uessellngh & LI nek & Tvrdlk109 Tek^ Saulckl f. Onda129 Vagi i I not187 Year 1934 1965 1972 1973 1974 1972 1966 1970 1973 1925 1941 1959 1966 1970 1971 1973 1973 1972 1962 S Species Cone, H Order II SO 5-50xlO-4 1 3 2.5xlO"5 0 SO.'H.O 1.7xlO"3 0 1 1.9x10-3 o S02'H20 0.009 0 HSOj" 0.03-.09 1 IISO." .001-. 01 0 SO 2' 0.009 1 S032" .009-. 03 3/2 S032" 0.04-0.4 3/2 S032" 0.25-0.8 0 SO 2" 0.8 0.5-1.0 S032" 0.06 1 SO.2" 0.01-0.05 1 SO ,2~ .001-1.0 1 3 0 S032" 0.8 1 S032" 0.8 SOj2" 0.3-0.8 0 S032" 0.8 0 S032" 0.1-0.3 SO 2" 0.3-1 S032" 0.008 1 °2 Cone, H Order 2xlO"3 I 0 3-6xlO"4 0 4-8x10-4 0 1.2xlO'3 0 1.2xlO"3 l.lxlO-3 1-4x10-4 0 0-0.003 0 2-llxlO"4 1-2 ,3-2xlO"3 2 1-8x10-3 1 l.lxlO'3 l.lxlO"3 1-7,10-* \ l.lxlO"3 1 1-11x10"* 2 6.4xlO"4 1 SxlO-* 2 l.lxlO"3 2 .3-3xlO"3 1 2 2-llxlO"4 2 1 l-15xlO"4 1 Cat Ident Cone, M Order Mn 3xlO"6-8xlO-5 2 2.7x10-4 2 Mn 1.8xlO-3-0. 18 2 Mn 1.8-9x10-5 2 MB 0.036-0.82 0.7 - Mn 6.6-19.9X10"6 l Co 10"7-3xlO"6 1/2 Cu ID'4 i Co 0-10° 1/2 C" 1°~3_5 .3 t Co 4x10 -10 Cu 10"5-10"3 1 Cu 10-9xl0'4 1 Cu .5 Cu.Veraene 10 Co 10"3 Co 4x10 * 1 Co 5xlO"6-10"3 1 Co 8.8xlO"6-10"3 1 Co 5xlO"7-10 6 1/2 Co 10"9-5xl06 .7 Co 0.5-7.0x10 1 Contacting Method 6 1 i r r 1 n« stirring II «. R "T" stirrinB stirring stirring H & R II & R stirring stirring Pack col stirring stirring low turb high turb Wet wall Met wall Stirring Wet Wall Wet Wall Wet Wall Pack col bubbling Temp 40°c l-s°r 25°C 25°C 25°C 25°C 25°C 25°C 25°C 35°C 25"c 10°C 25UC 25°C 30°C 30°C 30°C 25°C ^°r 30°C 25°C 30°C 20°C pll 7 5 - - 1-2 1-4 1.1 -10 - 8-9 9.2 B.I 10 R.7 9.2 7-9 8.5 7-9 7-9 10 8.5 8-9 k - m O7i in-7 ole .03-22.1x10 j gec 1.3-16x10-1° f-i^ n i i it ,n-lmole 0.3-2.46x10 ^ se- 1.66xlO'5sec"1 ,~-R m 0.053s*c-l l "C > 7 nr^in-5 mole 3.2-66x10 j"sec 1200 1/g mole sec 19.3 sec l - - 0.013 sec * 2.5x10° I/mole sec - 50 104sec"1 - 1-5x10* sec"1 1.43x10 I/mole sec 2.3xl06 I/mole sec - -i.Ar52^ s o l-sec +.4 l+1.46xlO"7 Cs S 0 Comment A impurity likely Ea 32^4^. obs. mass trans ll.il"01* inert apparat u«, Dl)l gave great care to purity of mat'ls. Ea 18.7 kcal/mole Ea 17.5 kcal/mole Ea 18.3 kcal/mole no effect due to stirring st hip.h speed stirring changed 02 order NH^ present no effect of added acid inh drops rate to 10"s Versene likely effect on mech Cat solubility exceeded Ea 12 kcal/mole Ea 10.53 kcal/mole absorp- tion Indep of hydrodynamics 0 order change sharp Ea 15 kcal/mole added SO, : no effect Ea 12.4 \cal/mole 0- order switches with Cat Cone 0, order change pradual
------- define the main kinetic questions. The only problem comes from the varying conditions in the experiments which render some data uncomparable. The order in sulfur is usually zero or one, but this depends on the pH range. For low pH the HS03" order seems to be 0: at high pH the S032" order may be one. There are also changing order results. The oxygen order is the most controversial and has the most complicated fluctuations. The order has been observed to shift from zero to two as the sulfite concentration changes (12), switch between one and two as the catalyst concentration increases (148), and to vary as its own concentration changes (129). Whether the change is gradual (129) or abrupt (109) is a question. Catalyst dependence is stronger than that of the reactants, but the value is unsettled. Mn and Co produce orders of magnitude change in the reaction rate by variations of only a few ppm in their concentrations (48). Mg, con- versely, produces a sluggish, but steady, increase in the oxidation rate as its concentration changes over orders of magnitude (42). Experiments on the pH are scattered widely in the literature. The treat- ment by Fuller and Crist is worthy of note (66). (Linek and Tvrdik also treat the subject well). (109). Because the effect of pH is so closely linked with the sulfurous solution equilibrium, no clear chemical trends emerge. At extremely high pH ( >12) , however, the rate is depressed (19, 115, 190). This behavior is sometimes due to reduction in oxygen solubility (118). Never- theless there appears to be a genuine chemical effect, for the rate reduction occurs as well for oxygen already in solution (101). The energetics of the reaction are not as fully examined as the kinetics. All the works report operating temperatures between 20°C and 40°C. The low pH activation energy is around 20 kcal/mol, at high pH it is near 15 kcal/mol. 16
------- The published rates vary widely in first and second order kinetic equa- tions. The values appearing in Table 1-3 are for conditions of low pH where half-lives of a day are common as well as instances of high PH reacting to half concentration in 0.01 sec and less. In the low pH reaction, the stirring also causes discrepancies. Schultz and Gaden (155) report a decreasing reaction rate with increased stirrer speed. This finding touched off studies (131) that have still not resolved the ambiguity. Also at low pH no true (86) induction period has been ob- served, although start up lags due to physical causes do occur (152). At high pH an induction period up to 2 sec is possible (48). The above questions all touch on the molecular activity making up the reaction mechanism. And, just as these specific questions remain open, the final elucidation of the oxidation mechanism remains to be done. It is gener- ally accepted (66) that the oxidation proceeds by a free radical chain- with or without metal participation. Many aqueous sulfur radicals possibly in- volved are characterized (190). The early suggestions by Haber (76) and Titoff (171) have given way to the proposed mechanism of Abel (2, 3, 4) and Backstrom (14, 15, 16). A few points of interest to all mechanisms are: the oxygen atom transferred does not come from the solvent (186), the uncatalyzed reaction occurs by initiation other than by stray metal ions (66), the acti- vation energies in the catalyzed reaction agree closely with those for some metal-ligand substitutions of the catalyst (108). The results of some recent oxidation studies in slurries have given better definition to the oxidation problems. Ramachandran and Sharma proposed a model for gas absorption in a three phase slurry system. An instantaneous chemical reaction was assumed to occur in the liquid boundary layer surrounding the solid particles. Two cases were 17
------- examined in their model. The first case assumed that solid dissolution was unimportant in the rate of gas absorption. The second case assumed that solid dissolution into the liquid phase was an important factor. Results from the second case showed the rate of gas absorption to be proportional to the square root of the concentration of the solids present. Bjerle, Bengtsson, and Farnkvist (28) conducted an experimental examina- tion of CaC03 slurry oxidation in a laminar jet absorber at 25°C and 45°C and pH 8.5. In their weak (2%) slurry they found the S02 mass transfer coeffi- cient to be nearly that in an otherwise identical clear solution (clear kS02 = 1
Items fixed or changed in Parts&Vendors releases 5.0.x
5.0.168
Fixes error when importing selected rows when multiple assemblies are specified in List Assy P/N column.
Fixes saving of List...Currency/Date Formats...Set Date Format.
5.0.167
PO and RFQ dates on printed reports were displayed with a fixed "short date" format. Now the date is displayed with the same user-set date format used elsewhere in the program.
Removes Portrait-only limitation of PO and RFQ reports so custom report layouts (only) can use a landscape orientation.
When changing the Type of an item created within an ECO to PL or CAT (on the Edit Item window), the parts list may now be edited (on the Edit List window) to permit the assembly definition to be built entirely within the ECO.
When changing an item created within an ECO to a MadeFrom item (on the Edit Item window), the MadeFrom relationship can be edited (on the Edit List window) to permit the item definition to be built entirely within the ECO.
5.0.166
Restores mutual exclusivity between CAT/PL (list types) and parent P/Ns on the item master pane controls.
5.0.165
Sets report module to work with previously saved custom report layouts.
Avoids update error message when adding item to ECO that has zero-length string in PNNotes field.
5.0.164
When 'Switching Manufacturer Name for Selected Items' or 'Switching Vendor Name for Selected Items,' all selected rows were not being transferred in one session. Now they are.
5.0.163
Fixes vertical lines showing on single row per item reports.
5.0.162
Sets Buy and Build pages to validate entry on the P/N dropdown, just as it does on other pages. ECO page dropdown now validates entry also.
Fixes CalculatePOTotals error message caused when auto-generated RFQ/PO is created with Tax2 setting of "Apply to Subtotal + Tax1"
5.0.161
Printing from the Assy Tree now accommodates up to 20 sub-levels, increased from 14.
Fixes Cost Summary Tree for very small cost values (subassemblies with total costs of only fractional cents were being rounded to zero).
5.0.160
Fixes 'Primary Only' selection when generating RFQs from a Purchase List.
5.0.159
Applies saved report layout to RFQs and POs when printing a group of documents from the Buy page.
5.0.158 (Service Pack 3)
Changes sort of parts list reference designators to selected rows only.
5.0.157
Fixes missing-last-column problem in export from Assy Tree, introduced in release 5.0.153.
5.0.155/156
New feature to sort parts list reference designators. Menu: List...Reference Designators...Sort.
5.0.154
New Import option permits user to specify separating reference designators with comma+space or space only. Flyout from Tools menu.
Importing was not saving the 'Item Stock' column value if the item's Status was Released. 'Item Stock Add' and 'Item Stock Subtract' functionalities were unaffected. Imports to refresh Item Stock are now independent of item Status.
5.0.153
Adds new export column from Assy Tree, showing parent assembly for each item (sometimes called 'Next Assy'). Active for exports to CSV or Excel.
5.0.152
Adds new File...File Utilities...Maintenance menu item: 'Refresh PLL only before needed' . This causes an internal refresh of assembly relationships to execute just before needed in Purchase List and Kit List compilation instead of occurring during other operations. May save time in some large database installations.
5.0.151
Import utility using 'List Assy P/N' column (for importing multiple assemblies) was assigning wrong title for the assembly if listing did not also contain the companion line item for the assembly item itself. (Corrected a bad response to sloppy import data.)
5.0.150
Fixes overflow message when opening POs and RFQs in very large data files.
5.0.149
Receiving decimal order quantities on a PO was sometimes resulting in a slightly different value due to data type conversion.
5.0.148
Changing the Status of an item to Unreleased now immediately enables the Parent, MadeFrom and Controlled checkboxes on the Item Master, which previously required saving and refreshing the record to enable them.
The input box for changing the Vendor name now has additional text to clarify its purpose: This is for fixing the spelling of this name only. To change Source to another vendor, be on the Vendor...Items Supplied grid and use the menu item List...Switch Vendor Name for Selected Items.
Same as above for changing a Manufacturer name.
5.0.147
Parts lists printed from the ECO Edit List window now have the option of appending the ECO number onto the report title.
PO total was sometimes showing rounding error for sum of high resolution subtotal and taxes, resulting in least significant digit being off by one cent.
If the default report layout file for a listing becomes corrupted (error 5711), the program will now unhook from that (.rpx) file and inform the user that it is doing so.
If attempting to close the Permissions dialog with no user set as PVAdmin, program prompted to fix this, but closed the connection to the database so that entry couldn't be fixed. Now works correctly.
5.0.146
Items in QuickBooks that are subitems of others are now transferred into P&V without their parent items. Example: Item ABC:DEF from QB is now transferred into PV as item DEF.
'Copy Selected to Another List' action is disallowed if the target list is on an open ECO or its Status<>Unreleased.
5.0.145
On the Add Item dialog, a fast-find entry was not always causing the grid to scroll to the relevant item.
5.0.144
When importing multiple assemblies in one session, some installations would not successfully complete the operation, producing an error message that referred to an OLE or Memo field.
Multiple assembly imports would leave Title = "Imported List" and Detail = "From file <filename> unless the overwrite box was checked. Now, if new destination assemblies have a line item in the import list, then the Title and Detail fields are written from available data.
5.0.143
Fixes false barcode status message 'FAILED' when scanning inventory adjustment on item master page that does not match input. Scanning from any other tab/page did not result in false 'FAILED' message.
5.0.142
Fixes error message upon attempting to import new/replacement vendor information for an item.
5.0.141
When importing multiple lines of the same P/N to add or subtract from Stock (columns 'Item Add Stock' or 'Item Subtract Stock'), quantities are now combined for the operation.
When importing multiple lines of the same P/N to set the stock quantity (column 'Item Stock Qty'), the last instance now overwrites previous instances (subsequent instances were formerly ignored)
Typing fast-find entries on the Import 'Set P/N for Selected Rows' dialog were causing an error message. Picking from the dropdown list was not.
5.0.140
Extends expansion of item master Revision field to 10 characters into ECO functions (was limited to previous 4 character field size). Run UpdateFileFormat.exe to set new field sizes in data file.
5.0.139
Fixes situation where entering foreign vendor costs on an RFQ would save incorrect value into displayed Cost on Vendor...Items Supplied grid (saved price breaks remained correct).
5.0.138
Editing the BuyNet quantity on the Purchase List will now cause program to re-look up the price break and extend costs on that grid.
If default PO notes file is missing, program was locking up when auto-generating POs. Now it shows a message that describes the problem.
5.0.137
On Compare Parts List window, menu item 'Print to PDF' was opening the report layout editor.
Fixes missing local settings file on failed compact.
Full setup file implements /NOFONTS command line option.
5.0.136
MadeFrom relationships were being stripped from items when released on an ECO.
5.0.135
1-Click checkbox was obscured on RFQ page enhanced dropdown when width was set narrow.
Cost Summary Tree column for Cost Each now has multi-decimal format consistent with cost each format elsewhere in program (3+ places).
Blank vendor entries, when importing, were being filled with vendor from previous row.
Program was permitting duplicate PO numbers
'Other' item was being checked for duplicates when being added to POs.
5.0.134
When making a Purchase List, the option of updating item and assembly costs was doing so even if the costs were set to zero (per cost option setting). Now zero values on the Purchase List don't update the item master.
Fixes ReadMe screen opening every time program is launched.
When making a Purchase List where 'Reduce by Qtys on Orders' was selected, and 'For Job#' was not, and PO line items for a part included a MIX of job numbers, the OnOrd column was less than the actual total on the specified range of POs.
Report title was blank when printing documents for selected rows on Pending RFQ(s) and Pending PO(s) grids.
5.0.133
Fixes hidden parts on Purchase List for German language Windows.
Fixes stuck caption "Total of Line Items - (No Tax)" on 'Totals By Job' report
On Assy Tree report, 'Hide Costs' option for sources subreport was hiding price breaks listing but not source line item cost. Now it does.
5.0.132
Enhancements to Vendor dropdowns on RFQ and PO pages (to be consistent with other control pane dropdowns):
-Keystrokes now cause open enhanced dropdown to scroll to matching item on the list.
-Tab keystroke now causes enhanced dropdown to close.
-Click on dropdown button when enhanced dropdown is open causes same to close.
5.0.131
Canceling Edit...Replace Item Everywhere was causing program to terminate.
Blank item numbers on parts lists were causing error on printout of tree.
5.0.130
P&V now flags QuickBooks' 15 character limit on ShipMethod and 11 character limit on PO Numbers when sending POs to QuickBooks.
Importing of Mfr and Vendor website URLs was causing an error. Now it doesn't.
Converting from a PV4 format data file was not updating certain cost fields from single to double precision, requiring running the separate UpdateFileFormat.exe utility. This function is now included in the conversion utility that runs upon opening the V4 database.
Converting previous version data files now sets currency format string field size to 35 characters to accommodate multi-character currency symbols.
5.0.129
Fixes initial conversion when first entering new costs in foreign currency on cost dropdown box.
5.0.128
Makes Vendor Leadtime field available for imported items.
Fixes error message when saving new ECO Archive report layout.
Fixes error message when removing a subassembly from the tree view.
5.0.127
New feature on Edit menu: 'Replace Item Everywhere' provides a way to substitute a P/N on all assemblies whose Status=Unreleased.
Import utility now includes 'Item Controlled' field when importing to the item master.
On Inventory Valuation Report, listing of items with cost=0 (tool to identify items without a valid source entry) was including items whose cost was not zero.
5.0.126
Import utility enhancements:
1- Now capable of bringing in multiple assemblies (parts lists) per session. Set column to 'List Assy P/N' to identify list.
2- New "Item Add Stock" and "Item Subtract Stock" column settings to handle inventory adjustments. Useful for processing CSV files generated from portable barcode scanning devices.
3- Now capable of saving multiple column layouts.Changes QuickBooks interface to send CurrentCost for all transferred items. This change requires specifying an Income Account on the Settings tab for Non-Inventory items (even though these items may never be sold).
Fixes "Primary Only" choice when generating RFQs from a purchase list.
Fixes missing last page when printing sheet style labels.
5.0.125
Fixes adjustment of Top Assembly inventory from Buy page (disabled in release .123)
5.0.124
Fixes entry of prompted ECO number (disabled in release .123)
5.0.123 (Service Pack 2)
Adds new Bar Code Scan input feature:
-- Find an item, Adjust Stock for an item, Receive an item.
-- Select the menu View...Barcode... or F11 key to open. Interface window is sized to be positioned below control pane on screens of 1024x768 or higher.
-- Responds to so-called "wedge" interfaces that send barcode scans as keyboard keystrokes.Adds barcode capability to Label printing.
-- Set any label line as a barcode.
-- If next line is set as blank, barcode will expand to use that space too.Fixes handling of comma used for decimal separator in various places in the program.
(PO Tax rate entries, fractional order quantities, and fractional Parts List and MadeFrom quantities when making Purchase and Kit lists.)Improved FastFind behavior on Add Item dialog when quickly typing characters (doesn't get ahead of itself).
On the Settings dialog, editing or adding new ship-to addresses were not being saved until clicking on another address row.
When editing the PO order quantity of an 'Other' item, program now does not attempt to look up cost.
On the PO Line Items grid, the Qty Rcvd column (cumulative quantity received) no longer accepts a blank entry (will revert to zero), ensuring a stock adjustment calculation will be made when items are received.
Fixes message "Cannot open any more tables" that was occurring on some systems after numerous control pane requeries.
Fixes error message when copying a parts list and responding 'No' then 'Yes' to copying sources.
Enhanced dropdown lists are defaulted to ON for new installations.
Updated interface DLL to QuickBooks accommodates QBSDK 1.1 requests from QBSDK 2.0 editions.
5.0.122
Fixes '13 - Type Mismatch' error message when attempting to edit the MadeFrom list of an MF item added to an ECO after Issued.
When Regional Settings are set to use thousands separator=. and decimal separator=, updating the exchange rate was resulting in syntax error message.
5.0.121
Fixes list width toggle (problem introduced in 5.0.120)
If set to transfer all items to QuickBooks as Inventory Items, program was still transferring items on POs as Non-Inventory items.
Fixes blanking of QuickBooks accounts when clicking the checkbox to show acct numbers.
5.0.120
Fixes misplaced screen splitter position when opening the program on some systems.
Pressing <enter> on Filter dialog was causing error if last user action was filling in match data.
Fixes errors when transferring POs to QuickBooks when the PO P/N has trailing spaces.
Fixes toolbar error reported on some systems when program opens.
5.0.119
Traps attempts to send P/Ns or Vendor names longer than the 31 characters allowed in QuickBooks .
Traps attempts to send POs to QuickBooks when PO Notes text exceeds QuickBooks' limit of 100 characters.
New checkbox option on QB Settings dialog to send PO anyway, without Notes.Fixes "Can't open any more tables" error when making a Purchase List on some systems.
5.0.118
New option to have the dropdown lists on toolbar for View History, Saved Views and Saved Filters expand to fill all available space. Right clicking on the toolbar background (between controls) will open a popup menu where you can make the setting.
New checkbox option to show account numbers when choosing QuickBooks item accounts.
Can now set a COGS (cost of goods sold) account for non-inventory items to be sent to QuickBooks.
Fixes accounts setting problem when connecting to QuickBooks Premier.
On PO Line Items, grid can now be sorted by (from P/N) column/cell.
Program now disallows direct entry of Current Cost on Item Master if there are existing Source records for the item.
5.0.117
Speed improvement on item search (for large databases) when typing into dropdowns on control pane, Fast Find on Add Item dialog, and column search boxes on enhanced P/N dropdown.
Notes indicators for Vendor and Mfr pages were not visible (as with PV4). Now they are.
Allows changing of grid column alignment by Alt+Clicking on column header.
Purchase and Kit lists were not being cleared on some systems when changing the Top Assy P/N. Now they are.
5.0.116 (Service Pack 1)
Fixes "Cannot open any more tables" error when making a Purchase List on some systems.
5.0.115
Permits showing 'OnOrd' quantity on Purchase List even if 'Reduce By' is unchecked. Set Pending, Open, Closed and Job checkboxes to specify scope of orders before unchecking 'Reduce By' checkbox.
New option to permit items with negative quantities on the Kit List. Make setting on Edit...Settings...Purch/Kit List. (Requested by user to apply "adjustment P/L" to a build without changing P/L quantities for base assembly.)
Changes caption 'Pending' to 'ToPrint' on PO and RFQ ShowAll grids and BUY Pending PO(s) and Pending RFQ(s) grids. Change made to avoid conflict elsewhere with Pending definition=DateIssued Is Blank and DateClosed Is Blank.
5.0.114
Fixes saving of default report layouts to disk files.
Fixes default paths to saved report layout files.
Saves license information to both local machine and current user registry areas to accommodate multiple Win2K logons.
5.0.113
When sending vendors to QuickBooks, vendor name is now copied into line 1 of vendor address, per QB internal behavior (so it shows on line 1 of purchase order address block).
Fixes duplication of last vendor address line on purchase orders sent to QuickBooks.
Fixes "5 - invalid procedure" error when sending purchase orders to QuickBooks and when listing PV Items on the 'Connection to QuickBooks' dialog (both due to existing items in QB where Name<>FullName properties).
5.0.112
On Win2k and NT, avoids "License Check File Not Found" message under some non-admin logons.
AVL (Approved Vendor List): The source field "Choice" is now available as a column on the Item Master Show All + Sources grid. A filter can be made for that grid where Choice Is Not Blank. If the Choice cell on the Sources grid has an entry, it identifies an approved vendor for the item. Further, if the grid's secondary sort (shift+click) is the Choice column, the vendors will be listed in the order of preference.
Adds "Leadtime" field to the assy tree listing.
On the PO Line Items grid, the cell "(from P/N)" is now available for printing on labels.
5.0.111
Fixes "Row handles must be released" message.
5.0.110
Fixes conversion of version 2 data files.
5.0.109
Fixes Filter error on enhanced dropdown when entering in multiple columns not including the last column.
Fixes conversion of previous version file format that prevents foreign currency values from showing.
5.0.108
Prevents Group License from being marked as invalid if entered multiple times in Help...About box.
When creating a new RFQ, default tax settings are saved with it, in case the RFQ is later converted to a PO.
Upon creating new PO, tax rate boxes on list footer continued to display rate from last displayed PO, until refreshed or items added to the new PO. Boxes are now blanked until refresh or adding items.
5.0.107
Special use only - Group License Fix
One of our users discovered that if you enter your group license number multiple times from the Help...About box, it's set as invalid. This Utility will clear that situation.
Method 1GroupFix
Method 2GroupFix
(1.1 megs)
(Version will show as ...107 or later, but it doesn't substitute for getting the latest update unless you already had ...106. Latest update 5.0.108 or newer will prevent the situation in the future, but won't clear the existing license invalidation.)(Both links retrieve the same file. Use the one that works with your browser.)
5.0.106
New Search and Filter feature on Item Master enhanced dropdown makes it easier to find desired part.
Printer selection dropdown list added to Page Setup dialog. Saves specific printer selection for each report type. Most helpful when one printer has 11x17 paper capability.
Duplicate Labels can now be printed from the PO Line Items grid, with label count = PO Order Qty. (Set the checkbox on the Label dialog.)
Changing a vendor's currency now prompts user to make cost value adjustments for cases where the foreign vendor's costs were entered directly in the foreign currency. (Example: Canadian organizations who entered their US vendors' costs in $USD instead of $CAD.)
"Saved Views" dropdown list was reverting back to that caption, preventing deletions of actual saved views.
5.0.105
Fixes error message when creating a filter that includes the Type field.
Filtering by the Type field on a Purchase List was not showing the dropdown list of available choices.
Fixes error when setting the destination list in Import module on a non-ECO file.
Fixes BOF error when importing into an empty data file with no vendors, mfrs, mfr p/ns.
Fixes error when copying a PO to a different vendor.
Improved FastFind behavior on Item Master when quickly typing characters (doesn't get ahead of itself).
5.0.104
Enables report and label layout editor for DEMO edition.
Fixes use of ECO demo license in non-ECO file.
When moving grid columns, horizontal scrollbar was temporarily inaccessible until grid was refreshed.
When printing from ECO Edit List window, Type, Detail and Ref were not printing.
Changed ECO Edit List report title was not being saved.
Auto refresh would cause a tmr_Timer Type Mismatch error if program was idling on an empty listing.
Fixes error when creating new ECO if program opened on ECO page.
Fixes error when releasing an assembly on ECO where a removed line item was not deleted from the item master.
5.0.103
Prevents applying Filter that is invalid due to blank criteria box.
ECO Edit List window now shows the number of list items in the status bar.
Fixes situation where adding an item to a PO would show fractional quantity in scientific notation when prompting to increase quantity to a whole number.
Fixes "License Check File not Found" when program was installed on a Windows NT/2k/XP with Admin logon, and a new license number was entered from another logon.
5.0.102
- Fixed error when copying an RFQ or PO to a different vendor.
- Fixed condition where program was permitting an RFQ to be copied to the same RFQ number.
- Fixed error "Cannot find table GPREF; License missing; exiting program."
- Fixed error upon attempting to delete an RFQ or PO under certain conditions. (Both could still be deleted from RFQ/PO ShowAll listing.)
5.0.101
- Fixed error after closing Print Preview of Purchase List: "Cannot find table GPREF; License missing; exiting program."
- Fixed error on ECO Edit List window when adding or replacing line items.
- Fixed error in ECO when refreshing Edit Item records: "PNE.PNNotes cannot be zero length string."
- Fixed RFQ and PO where sometimes changing Units did not save line item.
- If unable to open local configuration file, program now replaces file with saved copy.
5.0.100 initial distribution release
5.0.99 same as 5.0.105
5.0.98 same as 5.0.104
5.0.97 same as 5.0.103
5.0.96 same as 5.0.102
5.0.95 same as 5.0.100
- When printing a group of selected documents from the Buy...Pending PO(s) grid, the currency format was not sent to the report.
Added menu item: File...Print...Use Currency Format...Local/Vendor to select currency format for this operation. - View History listing was putting prefix from previously visited page on entries for Buy or Build pages.
- New splash screen and Help..About dialog graphic.
- Replaced Beta introduction window with Upgrade introduction window.
5.0.94
- Add Item dialog was not changing context when switching between listings.
- Copying a PO was not refreshing the dropdown list with the new PO.
- If Purchase or Kit list data listing has been edited, now a prompt to confirm overwrite pops up before making new Purchase or Kit list.
- On computers with very high resolution screens (1600+ pixels wide) toggling between Preview and Design tabs on the Report Layout Editor was leaving a vertical band on the left side of the screen, obscuring image.
- Alignment Toolbar on Report Layout Editor was not set to show by default.
- New menu item on Report Layout Editor to Set (snap) Grid Interval.
- New menu item on Report Layout Editor to open the Help file.
- New checkbox on Dial dialog (from Vendor and Mfr tabs) to suppress "1" prefix when dialing or faxing.
- On currency dialog, local currency is now always the first row.
- Notes and Names buttons on RFQ and PO pages were swapped in position from PV4.
5.0.93
- Non-US installations now fully initialize the Local Currency format.
- Connection to QuickBooks updated to work with QB Enterprise Edition and QB 2003.
- Purchase Order (printed report only) was showing job numbers offset upward by one row.
- Kit List caused error if no vendors, manufacturers or mfr P/Ns in the database.
- New menu item: Page...Disable Dropdown Autofill.
5.0.92
- During setup, if "Transfer Settings" was checked, an error message: 'No Current Record' appeared. Saved Filters were transferred; Saved Views were not.
- If blanking the Cur.Cost box on the Item Master, saving the record caused a 'Type Mismatch' error.
- Added "Default Font" to the Grid and Tree settings menus.
- Currency dialog was reworked, making it clearer how to enter new default local currency and removing format prompts that were getting in the way of entering new format text.
- When adding an item to a list, if cancelled due to a duplicate already on the list, the grid now moves to that item, setting it as the current record.
5.0.91 - Initial Beta Release
What’s New in the Basic Inventory Control 5.0.113 serial key or number?
Screen Shot
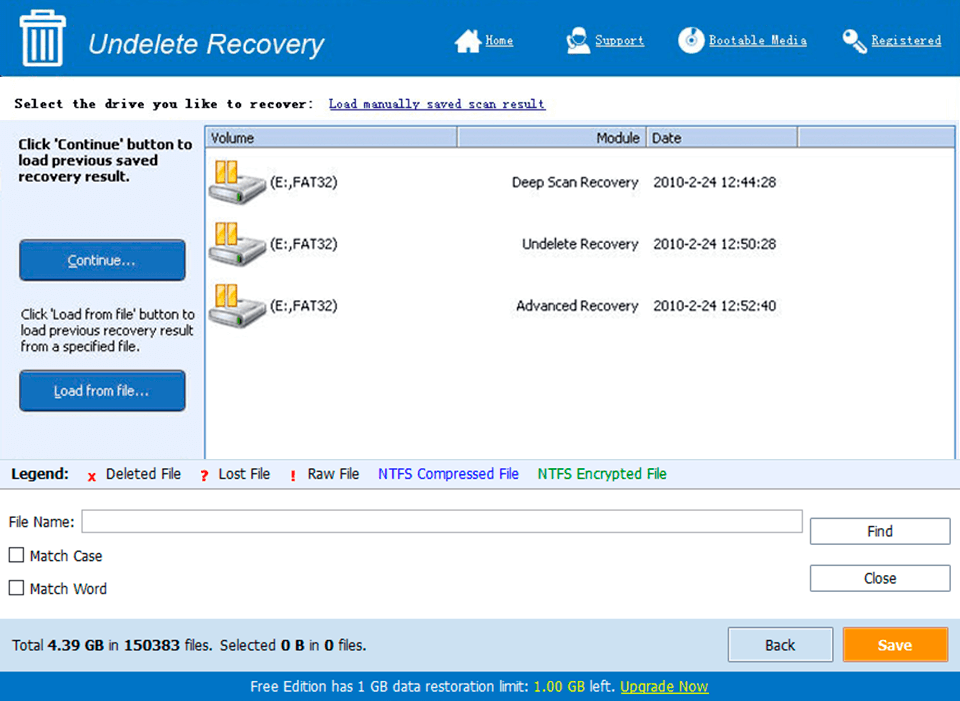
System Requirements for Basic Inventory Control 5.0.113 serial key or number
- First, download the Basic Inventory Control 5.0.113 serial key or number
-
You can download its setup from given links:


